We explain what the ozone layer is, and describe its characteristics and how to protect it. In addition, we discuss the ozone hole, its consequences, and more.

What is the ozone layer?
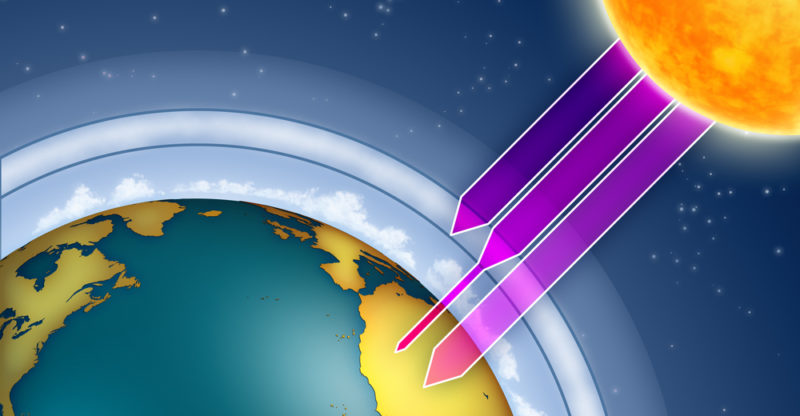
The ozone layer is a layer of Earth's atmosphere that contains a high concentration of ozone. It lies between 9 and 28 miles (15 and 45 km) above Earth's surface. It is also referred to as the ozonosphere.
By filtering most of the ultraviolet radiation that comes from the Sun, the ozone layer acts as a protective shield for the Earth. While a portion of this radiation is necessary for the development of life, high levels can be detrimental to living beings.
The ozone layer was discovered by French physicists Charles Fabry and Henri Buisson in 1913. In 1924, British physicist Gordon M. B. Dobson developed a measurement system to calculate the thickness of the ozone layer, called the Dobson Unit (DU).
- See also: Ecological footprint
Characteristics of the ozone layer
Among the main characteristics of the ozone layer are:
- It is made up of gas and is located in the stratosphere, the second closest layer to Earth's surface.
- It lies from 9 to 28 miles from Earth's surface (15 to 45 km).
- The ozone molecules that compose it are made up of three oxygen atoms (rather than two as in the oxygen molecule).
- It is essential for life on Earth as it filters out most of the incoming ultraviolet rays from sunlight, which can cause damage to living beings.
- Its thickness is measured in Dobson Units (DU), equivalent to 0.01 millimeters thick at standard pressure and temperature.
- The main chemicals that affect it are chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs).
- Through the Montreal Protocol, signed in 1987, all countries in the world committed to reduce ozone-depleting gas emissions.
Ozone-depleting substances

The main ozone-depleting substances are:
- Chlorofluorocarbon (CFC). It is a chemical compound containing chlorine, fluorine, and carbon derived from saturated hydrocarbons or petroleum. It is a major cause of ozone depletion, having the capacity to completely dissolve ozone molecules. Its use was widespread in all types of aerosols, solvents, and air conditioners, but nowadays it is rarely used.
- Hydrochlorofluorocarbon (HCFC). This compound is similar to CFC, but in addition to chlorine, fluorine and carbon, it contains hydrogen, which makes chlorine molecules more unstable when they come into contact with the ozone in the stratosphere. Today, it has virtually been phased out.
- Methyl bromide. This organic compound is used in agriculture for fumigation. It has been proven to be ozone depleting, though to a lesser extent than CFCs and HCFCs.
- Methyl chloroform or trichloroethane. This substance has been banned for causing chemical poisoning by inhalation and for depleting the ozone layer. It is an organic compound made up of a complex mixture of hydrocarbons, with a liquid structure and intense odor, which was used as a solvent in some industries.
- Carbon chloride or tetrachloride. This chemical gas has been banned due to its high level of toxicity to water, air, and soil. It was used in fire extinguishers and in the production of refrigerants.
What is the ozone hole?
The ozone hole is an ozone-depleted area in the ozone layer with a lower density or depletion of ozone molecules. This allows ultraviolet rays to slip through to the Earth's surface, which is harmful to living beings.
The causes of ozone holes are twofold:
- Natural causes. These encompass natural phenomena that may alter the concentration of ozone molecules. For example: volcanic eruptions, air currents in the stratosphere, solar activity, or low temperatures, which increase chlorine and bromine levels in the air and destroy ozone molecules.
- Human causes. These encompass human activity that depletes the ozone layer. For example: the use of CFCs in air conditioning and refrigeration, or the manufacture of plastic foams and aerosols.
Starting in the 1970s, scientists detected that the concentration of ozone molecules was decreasing in several areas of the ozonosphere, especially in the polar regions. This considerable decrease in the thickness of the ozone layer was termed "ozone hole".
Thus, with the aim of protecting the ozone layer, the Montreal Protocol was signed in Canada in 1987, an environmental agreement signed at the United Nations (UN) by representatives of various countries. It established a commitment to reduce the production and consumption of ozone-depleting substances at a global level, including CFCs and HCFCs. In 1994, the UN designated September 16 as the International Day for the Preservation of the Ozone Layer.
At present, the ozone layer is gradually recovering due to regulations implemented at the international level regulating the production and consumption of harmful substances. As a result, it is expected to be fully recovered by 2050.
Effects of ozone layer depletion
Ozone depletion has important consequences for life on the planet:
- Impact on human health. Chronic and overexposure to ultraviolet (UV) radiation can have negative effects on human health, causing skin cancer, premature aging, DNA damage, and weakening of the immune system.
- Sea life degradation. Marine organisms like phytoplankton, zooplankton, corals, and fish larvae, are particularly vulnerable to UV radiation. Overexposure can threat their health and survival.
- Impact on terrestrial vegetation. Terrestrial plant life may also be affected by overexposure to UV radiation, as it may cause alterations in photosynthesis and damage to DNA.
- Effects on terrestrial wildlife. Terrestrial animal life including insects, amphibians and reptiles, may also suffer damage, mainly to the skin and eyes.
Explore next:
References
- Casatti, P. (2017). Cómo afectan los rayos ultravioletas a las plantas. CONICET. https://www.conicet.gov.ar/
- Milo, A. (2023). Atmósfera terrestre: así funciona el manto vital que permite la vida en la Tierra tal como la conocemos. National Geographic en Español. https://www.ngenespanol.com/
- Nuñez, C. (2020). ¿Qué es la capa de ozono y por qué es importante?. National Geographic. https://www.nationalgeographic.es/
- Tarbuck, E. y Lutgens, F. (2005). Ciencias de la Tierra. Una introducción a la geología física. Pearson Educación.
Was this information useful to you?
Yes NoThank you for visiting us :)